Hap tip to Tamino:

Distributed Solar: The Democratizaton of Energy

Blogroll
- James' Empty Blog
- Lenny Smith's CHAOS: A VERY SHORT INTRODUCTION This is a PDF version of Lenny Smith’s book of the same title, also available from Amazon.com
- Dollars per BBL: Energy in Transition
- Mrooijer's Numbers R 4Us
- Thaddeus Stevens quotes As I get older, I admire this guy more and more
- Patagonia founder Yvon Chouinard on how businesses can help our collective environmental mess Patagonia’s Yvon Chouinard set the standard for how a business can mitigate the ravages of capitalism on earth’s environment. At 81 years old, he’s just getting started.
- "Consider a Flat Pond" Invited talk introducing systems thinking, by Jan Galkowski, at First Parish in Needham, UU, via Zoom
- Ives and Dakos techniques for regime changes in series
- John Kruschke's "Dong Bayesian data analysis" blog Expanding and enhancing John’s book of same title (now in second edition!)
- South Shore Recycling Cooperative Materials management, technical assistance and networking, town advocacy, public outreach
- Team Andrew Weinberg Walking September 8th for the Jimmy Fund!
- Darren Wilkinson's introduction to ABC Darren Wilkinson’s introduction to approximate Bayesian computation (“ABC”). See also his post about summary statistics for ABC https://darrenjw.wordpress.com/2013/09/01/summary-stats-for-abc/
- Why "naive Bayes" is not Bayesian Explains why the so-called “naive Bayes” classifier is not Bayesian. The setup is okay, but estimating probabilities by doing relative frequencies instead of using Dirichlet conjugate priors or integration strays from The Path.
- Peter Congdon's Bayesian statistical modeling Peter Congdon’s collection of links pertaining to his several books on Bayesian modeling
- Dominic Cummings blog Chief advisor to the PM, United Kingdom
- Charlie Kufs' "Stats With Cats" blog “You took Statistics 101. Now what?”
- John Cook's reasons to use Bayesian inference
- Rasmus Bååth's Research Blog Bayesian statistics and data analysis
- Flettner Rotor Bruce Yeany introduces the Flettner Rotor and related science
- The Mermaid's Tale A conversation about biological complexity and evolution, and the societal aspects of science
- Tim Harford's “More or Less'' Tim Harford explains – and sometimes debunks – the numbers and statistics used in political debate, the news and everyday life
- Simon Wood's must-read paper on dynamic modeling of complex systems I highlighted Professor Wood’s paper in https://hypergeometric.wordpress.com/2014/12/26/struggling-with-problems-already-attacked/
- What If
- Leadership lessons from Lao Tzu
- Mark Berliner's video lecture "Bayesian mechanistic-statistical modeling with examples in geophysical settings"
- "Talking Politics" podcast David Runciman, Helen Thompson
- Professor David Draper
- Earle Wilson
- Why It’s So Freaking Hard To Make A Good COVID-19 Model Five Thirty Eight’s take on why pandemic modeling is so difficult
- GeoEnergy Math Prof Paul Pukite’s Web site devoted to energy derived from geological and geophysical processes and categorized according to its originating source.
- Nadler Strategy, LLC, on sustainability Thinking about business, efficient and effective management, and business value
- Dr James Spall's SPSA
- Pat's blog While it is described as “The mathematical (and other) thoughts of a (now retired) math teacher”, this is false humility, as it chronicles the present and past life and times of mathematicians in their context. Recommended.
- Comprehensive Guide to Bayes Rule
- Fear and Loathing in Data Science Cory Lesmeister’s savage journey to the heart of Big Data
- Risk and Well-Being
- Mike Bloomberg, 2020 He can get progress on climate done, has the means and experts to counter the Trump and Republican digital disinformation machine, and has the experience, knowledge, and depth of experience to achieve and unify.
- The Keeling Curve: its history History of the Keeling Curve and Charles David Keeling
- Logistic curves in market disruption From DollarsPerBBL, about logistic or S-curves as models of product take-up rather than exponentials, with notes on EVs
- Woods Hole Oceanographic Institution (WHOI)
- London Review of Books
- All about models
- In Monte Carlo We Trust The statistics blog of Matt Asher, actually called the “Probability and Statistics Blog”, but his subtitle is much more appealing. Asher has a Manifesto at http://www.statisticsblog.com/manifesto/.
- Higgs from AIR describing NAO and EA Stephanie Higgs from AIR Worldwide gives a nice description of NAO and EA in the context of discussing “The Geographic Impact of Climate Signals on European Winter Storms”
- distributed solar and matching location to need
- Mertonian norms
- Carl Safina's blog One of the wisest on Earth
- International Society for Bayesian Analysis (ISBA)
- Busting Myths About Heat Pumps Heat pumps are perhaps the most efficient heating and cooling systems available. Recent literature distributed by utilities hawking natural gas and other sources use performance figures from heat pumps as they were available 15 years ago. See today’s.
- Karl Broman
climate change
- `The unchained goddess' 1958 Bell Telephone Science Hour broadcast regarding, among other things, climate change.
- Simple models of climate change
- Eli on the spectroscopic basis of atmospheric radiation physical chemistry
- The Keeling Curve The first, and one of the best programs for creating a spatially significant long term time series of atmospheric concentrations of CO2. Started amongst great obstacles by one, smart determined guy, Charles David Keeling.
- “The discovery of global warming'' (American Institute of Physics)
- "A field guide to the climate clowns"
- Nick Bower's "Scared Scientists"
- Interview with Wally Broecker Interview with Wally Broecker
- Wally Broecker on climate realism
- Steve Easterbrook's excellent climate blog: See his "The Internet: Saving Civilization or Trashing the Planet?" for example Heavy on data and computation, Easterbrook is a CS prof at UToronto, but is clearly familiar with climate science. I like his “The Internet: Saving Civilization or Trashing the Planet” very much.
- Andy Zucker's "Climate Change and Psychology"
- HotWhopper: It's excellent. Global warming and climate change. Eavesdropping on the deniosphere, its weird pseudo-science and crazy conspiracy whoppers.
- All Models Are Wrong Dr Tamsin Edwards blog about uncertainty in science, and climate science
- Ellenbogen: There is no Such Thing as Wind Turbine Syndrome
- SOLAR PRODUCTION at Westwood Statistical Studios Generation charts for our home in Westwood, MA
- Bloomberg interactive graph on “What's warming the world''
- Équiterre Equiterre helps build a social movement by encouraging individuals, organizations and governments to make ecological and equitable choices, in a spirit of solidarity.
- "Getting to the Energy Future We Want," Dr Steven Chu
- Climate Communication Hassol, Somerville, Melillo, and Hussin site communicating climate to the public
- Climate Change Reports By John and Mel Harte
- Jacobson WWS literature index
- "When Did Global Warming Stop" Doc Snow’s treatment of the denier claim that there’s been no warming for the most recent N years. (See http://hubpages.com/@doc-snow for more on him.)
- The Scientific Case for Modern Human-caused Global Warming
- Isaac Held's blog In the spirit of Ray Pierrehumbert’s “big ideas come from small models” in his textbook, PRINCIPLES OF PLANETARY CLIMATE, Dr Held presents quantitative essays regarding one feature or another of the Earth’s climate and weather system.
- And Then There's Physics
- ATTP summarizes all that stuff about Committed Warming from AND THEN THERE’S PHYSICS
- The Green Plate Effect Eli Rabett’s “The Green Plate Effect”
- NOAA Annual Greenhouse Gas Index report The annual assessment by the National Oceanic and Atmospheric Administration of the radiative forcing from constituent atmospheric greenhouse gases
- "Climate science is setttled enough"
- Climate Change: A health emergency … New England Journal of Medicine Caren G. Solomon, M.D., M.P.H., and Regina C. LaRocque, M.D., M.P.H., January 17, 2019 N Engl J Med 2019; 380:209-211 DOI: 10.1056/NEJMp1817067
- Climate at a glance Current state of the climate, from NOAA
- The net average effect of a warming climate is increased aridity (Professor Steven Sherwood)
- Klaus Lackner (ASU), Silicon Kingdom Holdings (SKH) Capturing CO2 from air at scale
- Climate change: Evidence and causes A project of the UK Royal Society: (1) Answers to key questions, (2) evidence and causes, and (3) a short guide to climate science
- Anti—Anti-#ClimateEmergency Whether to declare a climate emergency is debatable. But some critics have gone way overboard.
- Jacobson WWS literature index
- weather blocking patterns
- Ray Pierrehumbert's site related to "Principles of Planetary Climate" THE book on climate science
- Earth System Models
- "Lessons of the Little Ice Age" (Farber) From Dan Farber, at LEGAL PLANET
- Reanalyses.org
- Climate Change Denying Organizations
- Rabett Run Incisive analysis of climate science versus deliberate distraction
- The Sunlight Economy
- Documenting the Climate Deniarati at work
- Sea Change Boston
- Transitioning to fully renewable energy Professor Saul Griffiths talks to transitioning the customer journey, from a dependency upon fossil fuels to an electrified future
- MIT's Climate Primer
- Agendaists Eli Rabett’s coining of a phrase
- The Carbon Cycle The Carbon Cycle, monitored by The Carbon Project
Archives
Jan Galkowski




Dear mr.Blanket Man.
[snip]
The second law says that transfer of energy from cold to hot, can only be in the form of work.
Why does the gh-theory say that energy is transferred as heat to the surface?
[snip]
I don’t get many comments. shrug Getting comments or even traffic is not why I write my blog, but I do have the utmost respect for my 177 followers.
I’m leaving this one here as an example of what not to post.
Responding and engaging trolls are simply a waste of time that could be put to better purposes.
With the edits (made by the Moderator), the comment from @lifeisthermal now can be addressed in a proper context. The physical work related to the greenhouse effect is adiabatic expansion (where the “work” is coming from internal cooling) coupled with radiation of heat energy into space. The adiabatic portion occurs with constant potential temperature, at constant entropy. Lost of energy by emission is another energy exchange, indicative of a non-closed system. (Second law only applies to closed.) And the greenhouse effect and global warming are simply corollaries of the First and Second Law.
First note: Temperature of parcels of air decrease monotonically as altitude about Earth’s surface decreases. The rate of decrease is termed the lapse rate, and its profile is uniquely specified by any given atmosphere, whether it contains greenhouse gases or not. Atmosphere at higher altitudes is colder than atmosphere at lower ones, and the atmosphere at the surface boundary layer, in contact with the surface is the warmest of all.
Second note: The atmosphere, sans clouds and other scattering, is transparent to high frequently radiation from the Sun. It warms the Earth’s surface and, to the degree water is involved there (and pretending no water above) water in air near it. Because of the First Law, Earth must remain in thermal equilibrium with space about it. Since upon receiving a parcel of energy from Sun, Earth is incrementally warming because of it, this must be radiated out. And so it is, but the layers which radiate are high in the atmosphere, not near the surface. Thermal radiation is exchanged the dense layers by convection and collisions of molecules, and very little by direct radiation. Moreover, presence of dense layers of atmosphere above mean their cross section for thermal photos is very high. So they rarely escape.
Third note: Introduce, now, greenhouse gases in atmosphere. These are per unit mass much more opaque to thermal photons than ambient Oxygen and Nitrogen, and, so, impede the emission of thermal energy from any rising parcel of air. The effect is to change the lapse rate so high atmosphere at a temperature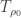 before the addition of greenhouse gas has
before the addition of greenhouse gas has 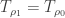 only when
only when  where
where 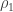 is the new altitude and
is the new altitude and  was the old one.
was the old one.
Fourth and final note: But if the lapse rate is such that the new equilibrium-by-thermal emission altitude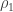 is higher than the original
is higher than the original  , that means, because the lapse rate is a monotonic decrease in temperature the temperature of atmosphere near Earth’s surface must necessarily be higher. So, it warms the surface more than it would without the greenhouse gases.
, that means, because the lapse rate is a monotonic decrease in temperature the temperature of atmosphere near Earth’s surface must necessarily be higher. So, it warms the surface more than it would without the greenhouse gases.
That’s it.